Titrating for Soaps in BiodieselTopics Discussed:
Why Titrate for Soaps?Titrating for soaps in your biodiesel can help you understand the success of your biodiesel process before and after washing. When used after washing, quite simply, it tells you how well you’ve washed your biodiesel. When used before washing, it gives you an idea of how close your process is to perfection and even more importantly, how much washing will need to be done, whether you use water washing or dry-washing. Supplies Needed for the Titration
The 0.01N Hydrochloric Acid SolutionWe make a 0.01N Hydrochlrocic Acid solution for soap titrations. We recommend using our already made solution since we make it in large batches, which leaves less room for error. If you purchase this, you can skip right to the soap titration. However, you may also prepare your own 0.01N HCL solution by using concentrated hydrochloric acid and distilled water. To prepare a 0.01N Hydrochloric Acid solution from concentrated hydrochloric acid, start by downloading our excel sheet on converting concentrated hydrochloric acid to a specific molarity. Next, you need to know the percent by weight of the concentrated hydrochloric acid you will be using. This usually does not change very much by the manufacturer, but it’s best to be sure what percent it is when you buy it. Check the current certificate of analysis for the hydrochloric acid’s concentration percent when you buy the hydrochloric acid, and make note of that concentration. To try and make your calculations even more accurate, we try to print links to the exact certificate of analysis for that particular HCL on the bottle. It is best to use that COA if available. Open the excel sheet and enter the percent of the hydrchloric acid in the "% Concentration" field. It is usually around 32%. Next, enter the "# of Mols in Solution" that you desire. In this case, you should prefer to do a 0.01N solution for this titration, but some will prefer to make 0.1N. Finally, enter the total volume of the solution (in ml) you will make into the "Desired Volume of N Solution" field. Usually, most users will make a 1 liter solution. In this case set the volume to 1000 ml. The Results:The excel sheet will tell you the volume or weight of the HCL needed to make the 0.01N solution. It is best to use the weight, since that’s easily measured with a pocket scale. If using our 1 liter bottles, place it on the pocket scale and tare/zero it. Use a syringe or pipette to carefully dispense the hydrochloric acid into the bottle up to the amount needed in grams. Since it’s usually around 1 gram, you may want to do only 1 drop at a time and try to get as close as you can. Try not to go over too much as you’ll need to rinse out the bottle, let it dry and start all over again if you get too much in there. Don’t worry if it’s off by a few hundreths of a gram. Remember that these pocket scales are very sensitive. After each drop, give it a moment to settle. It will initially read the force of the drop hitting the scale, and then the actual weight when it balances out. Dropping too fast often results in too much HCL. One you’ve put the right amount of HCL into the bottle, fill the rest of it up with distilled water to make exactly 1 liter. If using our bottles, 1 liter is to the bottom of the neck of the bottle. If you’re looking to be extremely accurate on the water, use a bigger scale sensitive to the gram, tare/zero it out and place the bottle onto it. Fill up to the amount of grams needed to make the 1 liter solution. 1 gram = 1 ml of water at 20°C, so you will need 1000 grams or 1 Kg of water to make a liter. The ml/g of water needed is also displayed in the results of the excel sheet for your convenience. If you’d like to be more accurate in making your solution, simply increase the volume of the total solution to be made. For example, 3000 ml solution instead of 1000 ml. The more HCL you need to dispense, the more accurate your solution will be. When using 0.1N Hydrochloric Acid In order to obtain 0.01N HCL from 0.1N HCL, you simply need to dillute it with water down to 10%. Add 10 ml of 0.1N HCL to 90 ml of distilled water and it will give you 100 ml of 0.01N HCL. This is what you will need for the soap titration. Testing for extra NaOH or KOH in unwashed biodieselUnwashed biodiesel can contain left over catalyst which throws off the results of the soap titration. Before titrating for soaps, it is best to test for this.
Note: Using a magnetic stirrer is much easier when performing titrations than stirring manually. Titrating for SoapsNow the 0.01N HCL solution will be added to test for the soap content of the Biodiesel.
The Math to Figure Out Soap in PPMTake the difference of the two weights recorded and that will be how many g of 0.01N HCL was added. Since the solution is basically water, 1 gram = 1 ml. The Equation: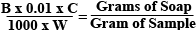 Where:
Multiply the final result of the equation by one million to get grams of soap per million grams of the sample. (ppm) The Easy WayThe easier way to obtain ppm is to just multiply the amount of 0.01N HCL used in the titration (difference in weight in grams or total ml used) by the catalyst factor and you get soap content in PPM. Using the Results for Dry-WashingMagnesolWhen using Magnesol, 1% by weight per 1000 ppm of soap should be used. The specific gravity of Biodiesel on average is about 0.860 so 8.6 grams of magnesol should be used for every 1000 ppm of soap per liter of biodiesel to be dry-washed. See below for the easy formula: (PPM of Soap) x (Liters of Biodiesel) x (8.6) / 1000 = Grams of Magnesol Required To get the most accurate results, it’s best to check the density of your biodiesel. Weigh out 100 ml of biodiesel on your pocket scale. Divide the weight by 100 to get the density. For example, if your 100 ml of biodiesel weighs 86 grams, take 86g/100ml = 8.60g/ml (common density of biodiesel) Ion-Exchange ResinsWhen using an Ion-Exchange resin, knowing the soap content of your unwashed biodiesel is important for maximizing the use of your resin. See our Ion-Exchange Resin Guide for more information. ASTM Standards & Understanding PPM of Soap After WashingThe soap titration can be performed again after washing to see if the biodiesel is of enough quality for use in your diesel engine. ASTM Standards call for no more than 41 PPM of soap when NaOH is used as your catalyst and 66 PPM when KOH is used. If the fuel tests at or below these standards, you have quality fuel. ASTM standards do not usually need to be met for clean operation, but are required for commercial use of the fuel. See the chart below for generally accepted values for home-brewers. Analyzing the quality of Biodiesel based on soap content chart
Fuel which is around or above 300 PPM of soap should be washed more before use. It is important to be sure your biodiesel is low enough in soap content to preserve your fuel filters and prevent longterm engine damage. |
||||||||||||||
|
Home | Customer Login | View Cart | Check Out | Forums | Contact Us | Affiliate Program
Heat Exchangers | Pumps | Filters | All Chemicals | Fluoroelastomer Hose (Like Viton Hose) Other Chemicals | Solenoid Valves | Stainless Steel Fittings | Stainless Steel Ball Valves | Check Valves Cone Tanks | Solar Water Heaters | Propane Conversion Kits | LED Lighting Magnetic Stirrers |
||||||||||||||
